 Subject: Physical
Science, Chemistry Subject: Physical
Science, Chemistry
Grade Level: 9-12
Materials: Computers with Internet access,
PowerPoint and Shockwave software, a computer with projector
and speakers (though individual computers can be used),
markers/colored pencils, paper, and the book Sophie's World by
Jostein Gaarder.
About: Using PowerPoint presentations,
online games, interactive experiment simulations,
debate/discussion, online audio recordings, and more, students
learn how the concept of the atom has evolved over time
Students complete a portfolio of work, including a
drawing/cartoon of atomic models, a lab report about
Rutherford's alpha-scattering experiment, and a creative
writing piece, as well as other work chosen by the
student.
Students learn how scientific knowledge changes and grows
over time. They learn through online interactives, classroom
and small-group discussions, drawings, creative writing,
computer games, PowerPoint lectures, and excerpts of
literature. Students become engaged in the material because
the ideas are big and every day brings something new to learn
and a new way to learn.
This unit is very thought-provoking for students. For
example, if atoms are conserved, then the atoms in my body
must be as old as the universe itself. I am 13.5 billion years
old! And if I am 99.9999% empty space, why can't I walk
through walls? Students see that scientific knowledge changes
with history and research, and realize that scientific
discovery is a process, not an end result. Teachers can
simplify or expand many of the lessons for their classroom.
Many activities can be used for middle school classrooms, as
well as high school chemistry classes. To make things easier,
I established a webpage where all materials are links.
http://nylearns.org/webpage/viewpage.aspx?ID=127702&UID=25397
| Students learn that scientific
knowledge evolves over time. |
| Students learn the basic atomic models:
Dalton's model, Thomson's Plum Pudding model,
Rutherford's model, and the Wave-Mechanical model. |
| Students learn to work in small groups
to debate, discuss, problem-solve, observe, analyze
data, conduct reseach, and develop presentations. |
| Students demonstrate their knowledge of
atomic theory through a variety of activities including
drawing, discussion, note-taking, hands-on acitivites,
online interactives, and writing. |
|
|
|
|
|
|
| This link is part of my
classroom/course website, and is the main page for
all other links. This page includes the PowerPoint
lectures, interactive lab simulations, audio
recordings of famous atomic scientists, online
games (Fling The Teacher, Atom Builder) and
more. |
| http://nylearns.org/webpage/viewpage.aspx?ID=127702&UID=25397 |
| This is an optional part of the
lesson. This link leads to An Elegant Universe
with many clips about atoms and the quantum world.
A Strange New World and The Quantum Cafe clips are
very good at showing students the complexity of
the atom and quantum laws. Students are engaged by
these clips and have MANY questions
afterwards. |
| http://pbs.org/wgbh/nova/elegant/program.html |
| Teachers may want to use a
stopwatch for the debate on Day One or other
activities. |
| http://online-stopwatch.com/ |
|
|
|
|
|
|
|
|
|
|
|
| Students demonstrate an
understanding of the structure of atoms. |
| 9-12 |
| Science |
| Students demonstrate an
understanding of big ideas and unifying
concepts. |
| 9-12 |
| Science |
| Students demonstrate an
understanding of the impact of science and the
impact of technology. |
| 9-12 |
| Science |
| Students use concepts from
Standards 1 to 4 to explain a variety of
observations and phenomena. |
| 9-12 |
| Science |
| Students use evidence from
reliable sources to develop descriptions,
explanations, and models. |
| 9-12 |
| Science |
| Students acquire information from
multiple sources. |
| 9-12 |
| Science |
| Students argue from
evidence. |
| 9-12 |
| Science |
| Students explain a scientific
concept or procedure to other students, and
communicate in a form suited to the purpose and
the audience. |
| 9-12 |
| Science |
| Students demonstrate scientific
competence by completing secondary research. |
| 9-12 |
| Science |
|
|
|
|
|
|
|
|
|
|
| Day 1: In the
beginning... |
| Students learn about the different Greek
philosophies regarding fundamental matter. |
| Students learn about the atomists,
specifically, Democritus. |
| Students read excerpts from the novel Sophie's
World by Jostein Gaarder. |
| Students work in small groups to read and
synthesize a particular philosophy, and share
their ideas with the class in the form of
mini-debates. |
|
|
| Sophie's World by Jostein Gaarder (excerpts
from pages 30-45) and The Natural Philosophers and
Democritus. |
| Markers/colored pencils and paper |
| stopwatch or go to
http://online-stopwatch.com/ |
| computer with projector and PowerPoint
software |
|
| Go over Homework 3 about the Greek Natural
Philosophers with students reading and giving
responses, or have students create a T-chart about
Ancient Greek Life vs Modern Life. |
| Remind students that life in ancient Greece
was very different than today (optional - show
students pictures of ancient Greek life). Have
students discuss some of the differences and how
this might change how people viewed their
world. |
| Let the class know about the many Greek
philosophies regarding fundamental matter. Give
each group of three or four a different excerpt
from the novel Sophie's World about a different
philosophy. Have all students quietly read and
highlight important parts of their excerpt. Next,
the groups choose roles and create
mini-presentations for their debate. During their
presentations, everyone participates: reading and
explaining a key quote from the excerpt,
summarizing the passage and overall philosophy,
presenting a drawing and explaining its connection
to their assigend philosophy, and making a final
statement as to why their philosophy is the best
one. |
| Have groups present and debate their ideas.
Discuss the different viewpoints. |
| Brief PowerPoint presentation about Natural
Philosophers. Go to www.nylearns.org/tredican
(Atomic Evolution) and then to Lesson 4: The
Natural Philosophers. |
| Students begin homework. |
|
|
|
|
|
| Students imagine that they are philosophers in
ancient Greece, and write a paragraph about their
own philosophy regarding matter (or Homework 4:
The
Alchemists). |
|
| Mini-debate/presentations |
|
| Day 2: What is Plum Pudding and what does
it have to do with the
atom? |
| Students learn about charges and the discovery
of the electron. |
| Students learn about the Plum Pudding model of
the atom. |
| Students learn how to interpret an
experiment/interactive diagram. |
|
|
|
| Computer with projector (or individual
computers), speakers |
| [optional] Plum pudding or fruit cake or
chocolate chip cookies |
|
|
|
| What does the phrase "opposites attract" mean
to you? Give an example. |
| Talk about Benjamin Franklin and charges. |
| Look at the interactive site about JJ
Thomson's experiment. Go to
www.nylearns.org/tredican, then to Atomic
Evolution for the Thomson electron experiment.
Have students relate their knowledge of how
charges behave to this experiment. Guide them to
the understanding that the charge of Thomson's
experiment must be negative. This charge was named
the electron. |
| Brief PowerPoint presentation about the
discovery of the electron. Go to
www.nylearns.org/tredican, and then to Atomic
Evolution to Lesson 10: The Story of the Electron.
Discuss the idea of Plum Pudding (maybe compare it
to a chocolate chip cookie). Optional: Bring in
plum puddding, fruit cake, or chocolate chip
cookies to help students visualize this atomic
model. I like to discuss how scienctists
frequently use everyday objects as a way to
understand new information. I ask students what
they think the JJ Thomson model would have be
called in modern American times. |
| Listen to a recording of JJ Thomson talking
about his discovery. Go to
www.nylearns.org/tredican (Atomic
Evolution). |
| Students write one specific scientific fact
that they learned today on a Post-It note; this is
their ticket to leave. |
| Students begin the next homework
assignment. |
| As students leave, they place their Post-it on
a posterboard about The Story of the
Electron. |
|
|
|
| Students research and write a paragraph, or
research and create an advertisement, about how
cathode ray tubes are still used
today. |
|
| Read and assess their discussion and
interpretation of the interactive presentation.
Read over their tickets to
leave. |
|
| Day 3: Why we should walk through walls,
and why we can't! [1-3 periods, depending on your class
needs] |
| Students learn about Rutherford's
alpha-scattering experiment. |
| Students use an interactive to "collect
observations/data" for their Rutherford lab
report. |
| Students learn important features of the atom:
empty space and the nucleus. The proton is also
mentioned. |
|
|
|
| Computers with Internet access and PowerPoint
Microsoft Word software, a projector, and
speakers |
| Colored pencils (optional) |
|
|
|
| Why do you think we cannot walk through walls?
What do you think can travel through walls? |
| Discuss some of the problems with the Plum
Pudding model. |
| Have students work in lab groups to use the
interactive lab simulation and to collect
observations/data. Go to www.nylearns.org/tredican
(Atomic Evolution) for Rutherford Lab Part II.
(Part I is for more advanced students or can be
discussed with the class as a whole group.)
Students can use colored pencils to draw
diagrams/observations. |
| Students work in lab groups to interpret the
observations/data. |
| Discuss as a class some of their
observations/results. |
| Students download lab report template and
complete individual (you can do group reports or
pairs) lab reports. They begin typing lab reports.
They finish for homework or next class. |
| Next class: Students finsih typing lab
reports, if necessary, and turn in lab
reports. |
| Brief PowerPoint presentation about the Story
of the Nucleus. Go to www.nylearns.org/tredican
(Atomic Evolution) to Lesson 11: The Story of the
Nucleus. |
| Go to www.nylearns.org/tredican (Atomic
Evolution) to listen to Ernest Rutherford
recording. |
| Talk about why we cannot walk through walls,
yet we are 99.99% empty space! Optional: Watch an
excerpt from An Elegant Universe. Go to
http://pbs.org/wgbh/nova/elegant/program.html
A Strange New World and The Quantum Cafe clips are
very good. |
|
| Finish Rutherford's Alpha-Scattering
Experiment lab
report. |
|
| Classroom/lab group discussions, lab
report |
|
| Students learn about nucleons [protons and
neutrons]. |
| Students learn about quarks, specifically up
quarks and down quarks. |
| Students learn what quarks make up protons and
neutrons. |
| Students use Atom Builder online interactive
game to build a carbon atom. |
|
|
| paper circles with +2/3 [quarks] -1/3
[quarks]. I usually color code them, and make
three of each kind for each group. |
| Computers with Internet access and Shockwave
software |
|
|
|
| Go over homework. |
| Review parts of the atom, particularly
nucleons. |
| Students learn that protons and neutrons are
made from up and down quarks. Tell them about
quarks. |
| Hand out the paper quarks. Have students use
the paper quarks to make a proton +1 using the
circles [two ups and one down]. Have students make
a neutron 0 charge [two downs and one up]. |
| Have the class talk about how to draw a model
of an atom. |
| Have students go to the Atom Builder
interactive game. Have them build a carbon atom.
They love this game, even though it can be
challenging! |
| Have students talk about the game experience
and what they learned. |
| Have students choose another element from the
Periodic Table. Have them draw a model of this
element, as their ticket to leave. |
| Students can begin next homework. |
|
|
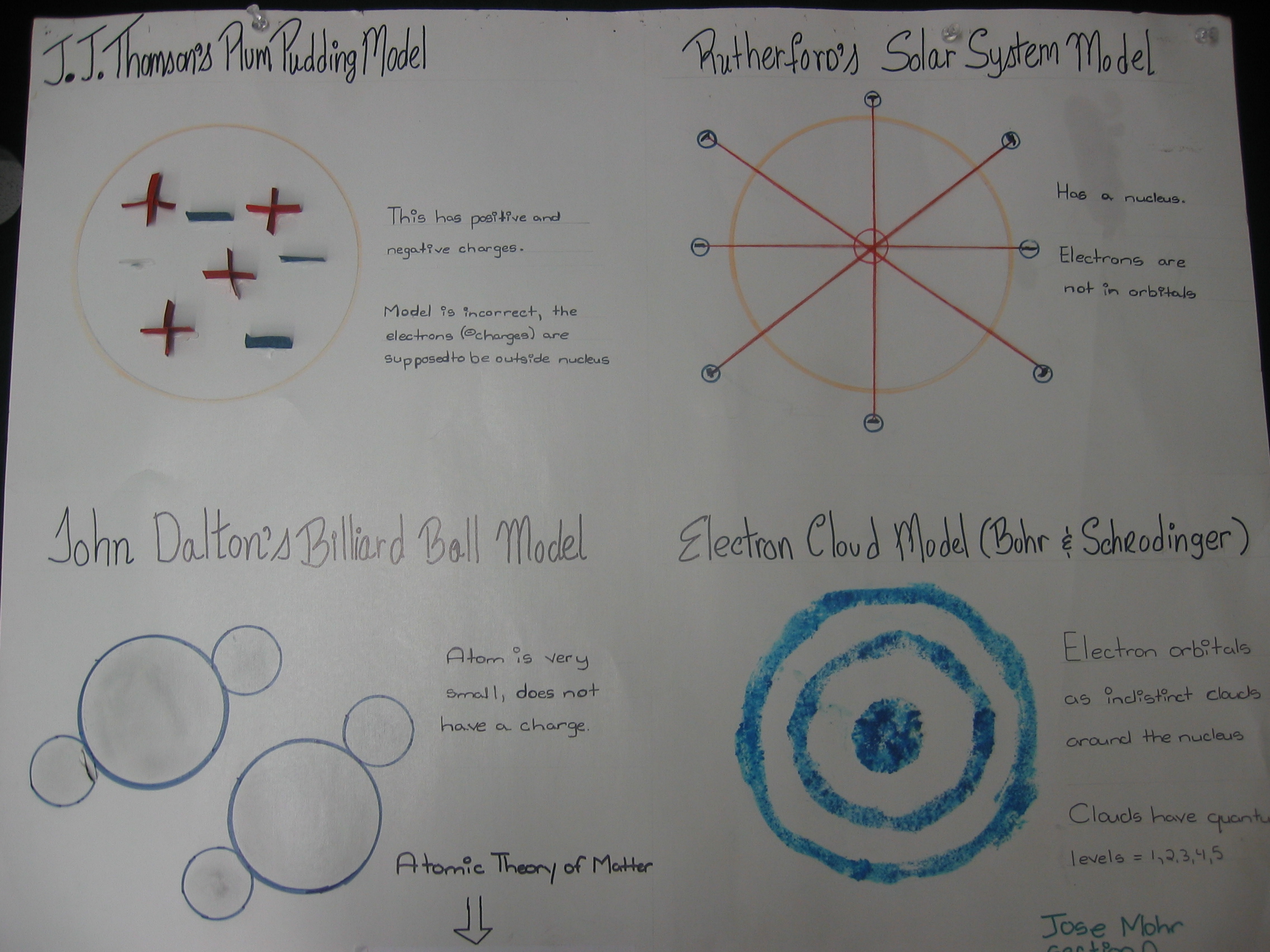
| Students draw pictures of all four atomic
models and explain the features of each
model. |
|
| Drawings of atomic
models. |
|
| Day 5: Putting it all together [3-4
periods, depending on your
class] |
| Students choose an atomic scientist for
further study. They work in small groups to create
a brief presentation [2-3 minutes] about their
scientist. Presentations can take the form of an
interview, news report, skit, poem/rap, or a
PowerPoint presentation, and should include a
visual component. |
| Students review their work to create a piece
of artwork and an artist's statement that reflects
one of the themes we have studied: Progress,
Discovery, Change, or Evolution. |
|
|
|
|
| Computers with Internet access, art
materials |
|
|
|
|
| Have students share and discuss their atomic
model drawings. Students discuss the evolution of
the atom and chemistry. The sharing of their
atomic model drawings can be done gallery-style or
a more traditional manner. |
| Students work in small groups. First, they
choose an atomic scientist they would like to
study further. They write five questions they want
answered. Each student goes online to find their
answer. Students come back together with their
information, and create a presentation based on
the class notes and their online research. They
create a script and a visual component. The brief
presentation [2-3 minutes] to the class can take
the form of an interview, news report, skit,
poem/rap, PowerPoint presentation, etc. |
| Students share their presentations. |
| Students work individually to create a piece
of art that reflects one the themes: Progress,
Discovery, Change, and Evolution. Students may use
PowerPoint or Inspiration or other software to
complete their artwork, or use more traditional
art media. After students complete their artwork,
they write an artist's statement. The first
paragraph explains how their art reflects their
chosen theme. The second paragraph explains how
the theme relates to the ideas studied in class. |
| Students play online review games to prepare
for the quiz on atomic evolution. Go to
www.nylearns.org/tredican to Atomic Evolution to
Fling the Teacher Game. Other review games can be
found at www.nylearns.org/tredican. |
| Quiz |
|
|
|
|
|
| Complete script and visual aide for
presentations, practice presentations, and
complete artwork and artist statement. |
|
| Presentations, artwork, artist statements, and
quiz |
|
|

Tara Redican
tararedican1@aol.com
Manhattan Village Academy
43 W. 22nd Street
New York,
NY 10010
Tara Redican began her teaching career in early childhood
education. After attending Bank Street College, she decided to
make the transition to the high school classroom. Applying her
background in chemical engineering, she has taught mathematics
and science at Manhattan Village Academy for the last seven
years. She strives to engage her students through an
everchanging variety of activities. One important part of the
chemistry course she teaches is the classroom website. All
PowerPoint lectures, review games, lab report templates, and
other resources are stored online for students, parents, and
other educators to use.
|